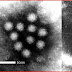
Picobirnavirus is a genus of dsRNA virus, which infect certain mammals. It may be implicated in gastroenteritis in animals and humans. The viruses have only been isolated from mammals to date.
The virons have a diameter of 35-40 nanometers with a triangulation number (T) = 1, 3 or 4.
The genome is a bipartate and double stranded RNA. The length of the two parts of the genome are 1.7 kilobases (kb) and 2.5 kb. The capsid protein gene is encoded by the second open reading frame of the larger genomic segment.
Picobirnaviruses (PBVs) are small, non-enveloped viruses with a bisegmented double-stranded RNA genome. Their pathogenic potential, ecology, and evolutionary features are largely unexplored. Here, we describe the molecular analysis of porcine PBVs identified in the intestinal content of dead pigs. Six of 13 positive samples were cloned and then subjected to single-strand conformation polymorphism analysis and nucleotide sequencing. All clones belonged to genogroup I PBVs and almost all clones clustered on separate branches from human strains. A single strain shared a notably close genetic relationship with a Hungarian human PBV strain (89.9 nt and 96.4 % aa identity). Genetic diversity was also observed among strains identified in mixed infections. Single point mutations and deleterious mutations within highly related strains suggested that PBVs exist as quasispecies in the swine alimentary tract. Clones with complete sequence identities originating from different animals suggested effective animal-to-animal transmission of the virus. Our findings indicate that infection with genogroup I PBVs is common in pigs.
Nucleotide and amino acid sequence identity data among selected human and porcine PBVs are available with the online version of this paper.
Picobirnaviruses (PBVs) belong to the newly proposed virus family, Picobirnaviridae /asp/iPublicMessageBoardMain.asp?Topic=5&MID=0&click=Vertebrate). They have a small, non-enveloped virion and a bisegmented double-stranded (ds) RNA genome; the large genome segment is 2.2–2.7 kbp long and encodes the putative capsid protein, while the small genome segment is 1.2–1.9 kbp long and encodes the viral RNA-dependent RNA polymerase (RdRp) (Chandra, 1997; Rosen, 2003). Based on the sequences of the RdRp gene, human PBVs are classified into genogroups I and II. The sequence similarity along a short nucleic acid fragment of the RdRp gene within and between the two genogroups ranges from 49 to 97 % and 28 to 37 %, respectively (Bányai et al., 2003; Rosen et al., 2000).
Laboratory diagnosis of PBV infections is mainly based on appearance of the two dsRNA genome segments in polyacrylamide gel separations. In spite of the relative insensitivity of this method, PBVs could be identified from faecal specimens of a variety of mammals and birds due to large amounts of virus occasionally shed through the faeces (Browning et al., 1991; Buzinaro et al., 2003; Chasey, 1990; Gallimore et al., 1993; Haga et al., 1999; Ludert et al., 1995; Masachessi et al., 2007; Pereira et al., 1988a; Wang et al., 2007). The development of virus-specific primers for RT-PCR amplification (Rosen et al., 2000) has been a milestone in the laboratory diagnosis of PBVs; however, thus far it has not been determined whether PBVs are pathogenic or innocuous agents of the intestine. A recent metagenomic analysis of faecally shed RNA viruses identified PBVs as a mixture of different strains in individuals without symptoms of gastroenteritis (Zhang et al., 2006). PBVs have also been detected in patients with gastroenteritis. PBVs have been frequently detected as co-infections together with rotaviruses, caliciviruses and astroviruses (Bányai et al., 2003; Bhattacharya et al., 2006a, b, 2007; Rosen et al., 2000). In addition, the higher detection rates of PBVs in immuncompromised patients without the detection of conventional enteric pathogens (Giordano et al., 1998, 1999; Gonzalez et al., 1998; Grohmann et al., 1993; Martinez et al., 2003) suggest that PBV might be an opportunistic pathogen.
The limited available information does not clearly establish an impact of PBVs on human health. Further, the lack of comprehensive sequence data does not allow establishment of firm epidemiological linkage between cases (Bányai et al., 2003; Rosen et al., 2000), or assessment of the potential existence of risk groups in the human population. It is also unclear whether the epidemiology of PBVs is influenced by host-species restriction or whether animals may act as reservoirs of infection for humans. Accordingly, gathering information on the genetic diversity of animal PBVs is critical to generate a more precise picture of the ecology of PBVs in humans. In this paper, a survey of porcine PBVs was carried out in order to obtain information on the genetic relationships between human and animal PBVs.
The intestinal contents of weaned pigs from various regions of Hungary were collected in 2005 as part of an ongoing programme aimed at investigating the zoonotic potential of known and recently emerging enteric viruses. Samples were sent with a diagnostic request by local veterinary practitioners to the Division of Pathology (Clinic for Large Animals, Faculty of Veterinary Science, Szent István University, Üllő, Hungary), where the gross pathological and bacteriological examinations were performed. A subset of samples was sent for virological examinations to the Regional Laboratory of Virology, Baranya County Institute of State Public Health Service (Pécs, Hungary).
Virological investigations included the following steps. Total RNA was extracted by use of TRIzol reagent (Invitrogen) from 150 μl 10–20 % suspension of faecal specimens (prepared in Tris/HCl, pH 7.2) following the manufacturer's recommendation. The RNA was resuspended in 60 μl DEPC-treated sterile distilled water (Bio 101 Systems) and frozen at –80 °C until analysis. First, 20 μl RNA was loaded onto a polyacrylamide gel and stained with silver nitrate to detect rotaviruses in the samples. However, only PBVs were detected by this method in 2 of 20 samples (designated C10 and E4). Of interest, sample E4 displayed four dsRNA segments in the gel with a size range consistent with that of PBVs (data not shown). To confirm these results, RT-PCR amplification was performed using the primers and the algorithm described previously (Bányai et al., 2003; Rosen et al., 2000). PCR products ∼200 bp in length were obtained in a total of 13 (out of 20; 65 %) samples. The uniform amplicon size suggested that all strains might belong to genogroup I PBVs (Bányai et al., 2003; Rosen et al., 2000).
For a subset of samples detailed diagnostic findings were available, revealing various scenarios of lesions in the organs and concomitant bacterial infections, that likely accounted for the death of the animals (Table 1⇓). Most importantly, in none of the PBV-positive animals was infection by PBV associated with peculiar clinical signs or pathology. Various health conditions associated with PBV infections have been reported by others (e.g. Cascio et al., 1996; Gallimore et al., 1995; Ludert & Liprandi, 1993; Wang et al., 2007; Zhang et al., 2006). In pigs, one study indicated that PBVs occur more frequently in diarrhoeic animals (Gatti et al., 1989), while another study reported that PBVs were detected at similar proportions in diarrhoeic and healthy animals (Ludert et al., 1991). Experimental infection of gnotobiotic animals would be required to acquire more conclusive data on the pathogenicity of porcine PBVs in piglets.
View this table:
Pathological diagnosis or gross lesion(s) and the bacteriological findings of PBV-positive pigs
+, Positive; –, negative.
To evaluate the relationships of Hungarian porcine PBV strains with other PBVs, nine samples were selected for sequencing on the basis of PCR product quantity and epidemiological context. The Big Dye cycle sequencing kit (version 1.1; Applied Biosystems) was utilized with the same primers used for PCR. Dye-labelled products were run and analysed on an ABI Prism 310 sequence analyser (Applied Biosystems). Visual inspection of the sequence chromatograms of all nine selected strains suggested the co-existence of heterogeneous amplicon populations. Therefore, six gel-purified amplicons were cloned into pGEM-T vector (Promega) and amplified in competent cells (Escherichia coli strain JM109). Depending on the clone numbers, 16–31 clones per sample were screened for PBV using the virus-specific primer pair, B25 and B43.
Positive plasmid clones were subjected to single-strand conformation polymorphism (SSCP) analysis in order to estimate the heterogeneity of the amplicon population and to select clones for further nucleotide sequencing. Briefly, 1 μl amplicon without purification was added to 18 μl molecular grade formamide (Sigma) and 1 μl 6× Blue/Orange loading dye (Promega). This mixture was heat denatured (97 °C, 5 min) and immediately placed on an ice slurry. The denatured amplicons (10 μl) were loaded on a pre-cooled polyacrylamide gel and were separated at 230 V, 50 mA for ∼100 min. Bands were visualized by silver-staining. Band patterns were categorized (Fig. 1⇓) and ≥1 clone representing each pattern was selected for nucleotide sequencing. Overall, between 4 and 10 plasmid clones obtained from the six selected amplicons were sequenced. Despite the optimized cloning procedure four clones were found to contain a mixture of DNA sequences and therefore they were not analysed further.
SSCP patterns and relative abundance of selected clones of six porcine picobirnavirus strains. 1, Sample identity; 2, no. clones subjected to SSCP; 3, SSCP patterns; 4, no. clones with the indicated SSCP pattern; 5, example clone. Clone names on the right hand side are identical to those given in the phylogenetic tree. The patterns of five additional clones are not shown because subsequent nucleotide sequence analysis revealed that four of them were mixed amplicon populations (in four cases) and one clone gave only faint bands in the gel (however, this latter clone yielded sufficient signal in the sequencing reaction). Asterisks indicate electrophoretic mobility of bands equivalent to ∼200 bp (*) and ∼400–500 bp (**). The molecular mass marker is not shown on the figure.
The resulting nucleotide sequences were edited and aligned with the GeneDoc software (Nicholas et al., 1997). The alignment included 43 porcine PBV sequences determined in this study and 17 human PBV sequences downloaded from GenBank, including the partial RdRp genes of three Hungarian, four Argentinean, one Thai, one Chinese strain, one gene sequence from India and seven gene sequences from the USA. Sixteen of these human strains belonged to genogroup I, while genogroup II was represented by a single strain. The Multalin free-ware (Corpet, 1988) was used to align longer gene sequences available in the DNA database. Phylogenetic analysis was performed by the neighbour-joining method with the p-distance model using the mega2 program (Kumar et al., 2001). A bootstrap resampling analysis of 500 replicates was performed.
Along with a short nucleic acid fragment of the RdRp gene (168 bp), the nucleotide sequence identity between any of the porcine PBV clones and those of human genogroup I strains ranged from 50.6 (e.g. E2-14 vs 745-ARG-95) to 89.9 % (E4-14 vs 1-HUN-01), while the range of similarity among porcine strains was between 54.5 (C10-5 vs D4-3) and 100 % (e.g. D4-5 vs D6-10). In these comparisons the nucleotide sequence identity values fell within the same ranges as seen among human genogroup I PBV strains (e.g. 49.4 % between 104-FL-97 and 745-ARG-95, and 97.6 % between 207-FL-97 and Hy005102). See details in the similarity matrix of human and porcine PBVs (Supplementary Table S1 available in JGV Online).
In the phylogenetic tree several clades supported with high (>90 %) bootstrap values could be distinguished (Fig. 2⇓). In a few cases complete and almost-complete sequence identities were identified among clones derived from distinct animals, suggesting that PBV strains can be easily transmitted from one host to another. All but one of the clones clustered on branches distinct from human strains. A single clone (designated E4-14) was most closely related to a Hungarian human PBV strain (89.9 nt identity and 96.4 % aa identity). Interestingly, the extent of sequence variation along the 168 nt fragment of RdRp correlates with the overall sequence variation of the entire RdRp gene for those two strains (1-CHN-97 and Hy005102, 61.9 % for the short fragment and 62.1 % for the full-length gene; data not shown) for which currently the complete RdRp gene sequence is available (Rosen et al., 2000; Wakuda et al., 2005). A taxonomic scheme based on partial RdRp sequences that are amplified with the broadly reactive primer set would be beneficial for future epidemiological studies on PBV, analogous to the genotyping systems used for other non-cultivatable small RNA viruses.
Phylogenetic relationship of porcine (po) and human (hu) genogroup I picobirnaviruses based on nucleotide sequence. In most instances, human and porcine strains separate into distinct genetic clades. In a single case, however, we identified close genetic relatedness between two heterologous strains (E4-14 and 1-HUN-01). Bootstrap values above 90 % are indicated. Bar, 0.05 substitutions per nucleotide.
The extent of sequence heterogeneity within an isolate has not yet been thoroughly studied for PBVs, although re-analysis of sequences from a metagenomic investigation of RNA viruses shed in the faeces revealed a heterogeneous population of PBVs in healthy individuals (data not shown; Zhang et al., 2006). A notable result of our study was the detection of mixed infections by different PBV strains in pigs. Furthermore, a remarkable genetic variability was observed within the RNA of the same PBV strain, likely accounted for by continuous accumulation of point mutations. A variety of these substitutions were nonsense mutations. We also identified a single point mutation in one clone (D6-1) that resulted in an in-frame stop codon within the RdRp gene. In another clone (C10-5) a deleterious mutation of three residues resulted in the removal of an amino acid but was not accompanied with the termination of translation within this short gene fragment (data not shown). Mutations altering the open reading frame may be tolerated in the presence of non-mutated copies of the virus genome, and able to compensate such deleterious mutations (Yoon et al., 2006). A preliminary investigation into whether the PBVs exist as a quasispecies was initiated, and data suggesting that the virus may exist as a quasispecies were obtained (unpublished results) but, for PBVs, these data and how the data were generated need to be studied in much more detail for reliable conclusions to be made.
PBVs are regarded as enteric viruses because all cases reported thus far have been associated with virus shed in the faeces and some data suggest that they may be associated with diarrhoea under certain conditions (Giordano et al., 1998, 1999; Grohmann et al., 1993; Pereira et al., 1988b). In this study we demonstrated the spread of a PBV isolate in the affected community (in a swine herd in this case), the co-infection of affected animals with several unrelated PBV strains, the possible quasispecies nature of this small dsRNA virus, and provided some evidence for a wider host-range for certain genetic clades of genogroup I PBVs. Although most porcine genogroup I PBV strains seem to form separate genetic clades from human isolates, the question whether host-species mechanisms exist requires additional gene sequences from these species to be analysed. Finally, our findings suggest the possibility that certain porcine and human PBVs shared crossing points in their evolution. Repeated exposures of humans to heterologous, but genetically related and rapidly evolving viruses shed in large amounts from domestic animals might be an occupational health risk that needs attention and thorough investigation in the future.
 Pbstealer.E tries to remove itself after sending data over Bluetooth. This self-removal doesn’t always work, but fortunately it can be also removed by uninstalling it with Symbian application manager.
Pbstealer.E tries to remove itself after sending data over Bluetooth. This self-removal doesn’t always work, but fortunately it can be also removed by uninstalling it with Symbian application manager.